MENU
In Ireland, a significant focus is being placed on environmental objectives within businesses, similar to the trend in the UK. The adoption of the Corporate Sustainability Reporting Directive (CSRD) is a crucial step in this direction. This directive mandates that large companies and listed SMEs report on their environmental, social, and governance (ESG) impacts starting from January 2024, with other larger companies joining in by January 2025, and listed SMEs by January 2026. This legislation aims to enhance transparency and ensure that companies disclose their environmental impacts and strategies comprehensively.
Securing green grants is a proven strategy to boost your success in achieving environmental goals. At CMP Solutions, we specialise in helping companies win these grants and maximise their benefits. Remember, by 2030, the carbon tax rate will reach €100 per tonne of CO2. Discover how green grants can empower your business to reach new heights in sustainability and environmental performance while avoiding increasing costs.
What Are Green Grants?
Green grants are a popular choice for businesses like yours across Ireland, thanks to government initiatives aimed at supporting companies dedicated to making a positive environmental impact. These grants provide crucial support to businesses to develop clear, actionable plans for sustainable improvements helping them turn their green ambitions into reality. Examples include introducing environmental best practices and structures, achieving cost and resource reduction targets, and laying the foundations for future environmental improvement projects. To learn more, check out our business grant application support service
How To Use Green Grants To Meet Your Environmental Objectives
The first step is to utilise a qualified expert to develop an improvement plan such as:
To help make these and other changes that can improve sustainable performance, your goals must be clearly defined and pre-tested. This highlights the importance of pre-grant application work. By working with your consultant to carefully define the environmental benefits of a project, the chances of success are higher. Knowledge is key to making sustainable changes, as is the ability to think outside the box and approach things differently.
Green grants can catalyse significant advancements in your operations by directly addressing key environmental challenges. Small changes can bring substantial benefits, whether upgrading to energy-efficient machinery or improving waste management systems. This approach enables your business to leverage a single grant to initiate continuous, impactful improvements tailored to your specific needs and scale.
How Can CMP Solutions Support?
At CMP Solutions, we have proven experience in identifying and developing projects guaranteed to improve performance. Our team can identify opportunities to enhance your environmental performance and offer tailored recommendations to optimise your sustainability efforts.
Ready to apply for green grants? Contact our team today, and let's elevate your business's sustainability together.
With more than one million companies worldwide being ISO 9001 certified, the accreditation continues to grow in popularity. Thanks to its versatility and detail-orientated approach, which can improve various sectors, the award has global notoriety.
As experts in supporting both manufacturing and process solutions, we have seen first-hand how ISO 9001 can improve productivity for various companies. In this article, we will share some information about what ISO 9001 covers, its benefits and how using a quality management system can elevate operations.
First published in 1987 by the International Organization for Standardization (ISO), the 9001 certification implements compliance measures for quality management standards. This covers processes, employees, resources, operations, data management, and financials, all of which are maintained via regular auditing.
QMS systems are essential for keeping standards high and mitigating risks. This can be the difference between success and failure within manufacturing and process industries. Another purpose is to meet customer demand and expectations by consistently producing high-quality services. Whether that is the creation of a product or service, keeping standards consistent is essential. ISO 9001 establishes the right processes for this via a set of regulations that each company must adhere to.
ISO 9001 does take some work from companies that wish to become compliant, but there are many rewards for investing the necessary time and money. These are as follows:
ISO 9001 is incredibly popular within these industries as they rely on succinct systems. The award can reduce risk, improve team structures and ensure efficiencies are incorporated into production systems. Our team are well versed in assisting with the ISO 9001 certification, so please contact us to learn more.
Reducing waste is an essential consideration for many businesses. It’s not only an essential sustainability factor but one that can directly impact the balance books with financial benefits awaiting forward-thinking companies. Even though organisations have access to various methods of reducing waste, making changes often relies on a financial injection that can facilitate such change.
At CMP Solutions, we understand that not all companies can make these amendments without an investment, so we offer Lean grant support. To understand how these Irish incentives can help you to maximise value and improve operating efficiency, keep reading.
The Lean grant is facilitated by Enterprise Ireland as a way to improve the overall business landscape within the country. Its purpose is to encourage companies to adopt tools and techniques that can improve operations.
The support was created as it was clear that many companies knew what changes they needed to make but lacked the financial injection needed to transform processes. As the money awarded is pivotal to success, we created our support service to ensure winning organisations get the most from their cash flow boost.
Lean grants were initially scoped out for manufacturing companies but are suitable for a range of other sectors, including software and production.
Lean by name and nature, the strategies that can be implemented for grant winners are designed to improve company efficiency, cost competitiveness and ongoing sustainability. They help businesses to streamline operations which can ensure waste is reduced and company value skyrockets. Waste is also an important aspect to consider as it doesn’t just relate to lost money but also to negative impacts on brand reputation that can be gained for businesses that do not operate sustainably.
Lean operating styles improve efficiency to create a culture of continuous improvement which runs more smoothly. This also ensures that certain aspects of performance can be automated allowing workforce to be used better by hiring companies. As well as reduced waste and better sustainability, other benefits also include better company culture, increased transparency across a company and operating methods that can be scaled when required.
At CMP Solutions, we have proven experience of helping a range of companies benefit from Lean grants. From food processing to distribution, our frameworks allow businesses to improve efficiencies that can lead to long term change. Our lean digital services highlight the methods we have chosen to track and make changes which are ideal for companies of all sizes and maturities.
This work also lends itself to our wider cost reduction and processing solutions, which see organisations improve quality, meet lead times faster and guarantee high output quality.
To learn more or book a free consultation, contact us now.
Engineering is an essential component of manufacturing, meaning streamlining directly impacts various measures of success. From saving money to enhancing productivity, manufacturing companies can make noticeable improvements with the right considerations for process optimisation.
Keep reading to learn how we believe the impacts of such improvements can lead to attainable successes at CMP Solutions.
Many companies have a range of goals to meet, which often rely on constant management from team members. However, by undergoing the process of process optimisation, these changes can be streamlined and enhance success rates.
Process optimisation for manufacturing uses proven methods of improvement and defined calculations to create the best processes for manufacturing systems. This means finding the optimal combination of input variables, such as material selection, processing techniques, and product specifications, to produce the best output. Businesses can choose their success factors, but productivity, cost and performance are the most common.
The impact of engineering optimisation has many effects on manufacturing with the main ones being as follows:
Companies can take the guesswork out of manufacturing results by understanding the ideal formula for improved outputs. This is achieved through the reduction in wasted time and improvements to how operations are completed. Furthermore, there is less chance of issues arising during manufacturing as bottlenecks have been accounted for and adjustments made. Finally, these processes also allow for future proofing to be considered as the same formulas for success can be applied to new manufacturing needs.
Process optimisation also ensures that manufacturing lines are efficient. By only using resources essential for outputs, environmental impacts are minimised. This also positively impacts areas such as waste disposal and brand reputation, as manufacturers can provide evidence of better performance levels.
One of the main reasons that companies should optimise process methods is to save money. By analysing manufacturing efficiencies versus required outputs, costs can also be budgeted with higher levels of accuracy. This is particularly useful when costs inevitably fluctuate, meaning a view of intended performance can still be viewed. Further ways to reduce spending are achieved by less resource consumption, optimisation of technology, and less waste.
Process optimisation improves employee management in two ways. Firstly, it means that accurate needs for workforce support are identified, improving the efficiency of budget spending on employees. Additionally, engineering calculations mean a skilled workforce is required, leading to more demand for these professionals. A common misconception is that employees lose work through process optimisation. The reality is that more jobs can be created as companies have the funds and knowledge to maximise the investment of good employees.
We offer cost improvement programmes that are proven to work. As outlined by the results you will see when using our cost improvement calculator, we have the know-how to help companies achieve a stronger ROI as well as utilise their workforce more effectively.
Get in touch to learn more.
Thanks to ever-rising supply prices; this task is essential for many as it’s often the only way to boost profits and ensure that projects end with positive capital. Reducing costs is also the key to remaining when other costs are spiralling, as not all companies will have the know-how to make sustainable and quantifiable change.
If you are looking for ways to efficiently reduce manufacturing costs to boost profit and brand reputation, you are in the right place. CMP Solutions offers business transformation services that allow the fundamentals of the operation to be overhauled with actual cost reductions.
The key to boosting profits through cost reduction is to make lasting changes. Short-term changes don’t often lead to long-term gain. Instead, new ways of working should be considered, which ensures that cost-reducing measures are embedded into every part of operations.
To make this successful within the manufacturing space, we believe the task should be broken down into overheads and automation.
This element of manufacturing operations often causes costs to skyrocket, as the associated prices are usually not within your control. While eliminating certain overheads, such as energy usage and premises costs, is not an option, there are ways to streamline spending to lessen the financial impact.
Some examples of this could be converting to new shifts when energy costs are lower, using energy-efficient machines in-house, or even allowing certain team members to work remotely if they don’t have to physically be always onsite. Another factor to consider are material and supply chain costs. Profit margins can be boosted if waste is reduced and operations streamlined to ensure attention is paid to lower cost materials which offer a better return on investment. To make all of this possible, we work with companies to create cost-highlighting processes, which means all overheads can be adapted to fall in line with cost models. Sometimes, all you need to adopt is clever thinking and shared understanding across a business.
Automation at scale is thought to be one of the most effective ways of elevating profits. Within manufacturing, this comes in the form of digital transformations, employee training and process improvements. Whether you select intelligent robots for the production line or bring software in-house that reduces manual admin duties, all solutions offer a way for companies to dramatically reduce spending.
The benefit of this is also that output can be increased, which leads to a wider profit margin.
At CMP Solutions, we have a proven track record of transforming manufacturing company operations to boost profits. This is achieved through support from our expert team and the implementation of proven results-driven measures.
The advice we have shared in this article can be applied to companies of all sizes. Furthermore, each should also be considered by understanding that streamlining and standardising work processes is an essential method of improving efficiency in terms of resources and time .
Remember, cost management is often as crucial as generating sales. For every Euro saved through efficient process management, there is a direct and full contribution to the bottom line. In contrast, each Euro earned in sales only contributes the margin to the bottom line. This distinction highlights the significant impact of cost savings. Effective cost management, through streamlining and standardising work processes, plays a pivotal role in financial health, often equating to or surpassing the benefits of increased sales.
Example:
Company A sells €2000 worth of product at 25% margin = €500 to the bottom line.
Company B reduces costs by €500 = €500 to the bottom line.
Which of the above is easier for your business to do - turnover €2,000 in sales or cut costs by €500? Of course, you need to balance both but many businesses actually increase costs by putting time and resources into getting sales that are ultimately detrimental to the business.
To learn more, contact our team or instantly receive an idea of how much we could save you via our cost improvement calculator.
Product (and/or process) lifecycle is the time from initial concept to retirement of a given product. There is a significant level of activity occurs in the intervening period, much of which happens before the product ever comes to market. There is a lot of literature out there that breaks this activity into four very broad categories; Introduction, Growth, Maturity and Decline. Some will add additional categories such as development, saturation etc. Rather than focus on categories, this paper will attempt to break down the product lifecycle into the relevant processes that a company must consider when planning to bring a new product to market. Elements of this paper can also be used when making changes to existing products.
Figure 1 below graphically illustrates the seven processes that form the product lifecycle. While this may look significant at first glance, there are an infinite number of variations on how it can be completed and the order in which it is done so. The level of timing and resourcing required for each process depends upon the given situation. For example:
The process illustrated above is iterative. At any point, you may discover that you cannot proceed without stepping back to the previous process and making changes. The remainder of this paper will look at each of these 7 processes in more detail.

Figure 2: Elements of the Design Phase, required before proceeding to Design Validation.
Design starts with an idea, a concept that solves a problem. This includes improving an existing product/process or developing a superior product. Once you have this, you should document all the key features, drawings, dimensions and anything else that is currently known about product. While this is important, don't agonise over it too much because you will find that you will make changes during subsequent revisions of the design process as you learn more.
This involves determining feasibility of the design from both a practical and marketing point of view - can it be built in a cost-effective way, does the market want it, who are the target audience, how much are they willing to pay for it etc? A prototype is a good idea at this point because it allows you to verify feasibility in a physical sense, solicit user feedback and get an appreciation of the visible attributes of the product. Finally, a patent search is important so that you do not waste valuable time, money and resources on your concept, only to later find that you cannot proceed with.
Complete your design evaluation. Once the design evaluation is complete, the design inputs can be generated. A thorough evaluation should include:
For highly regulated industries (e.g. medical device), the manufacturer is responsible for ensuring that the design inputs are appropriate in addressing user needs. Design inputs are deemed to be the physical and performance characteristics of a device that are used as a basis for device design and consequently, manufacturers are obliged to establish and maintain procedures to this effect. Design development is essentially about further improving the design.
As the name suggests, this phase is about testing and analysing the design. The result of which is the determination of the design outputs. A robust test method design is important here prior to completing any testing to ensure that a test method is suitable for its intended use. The test method will need to be validated and this will be discussed further in the design validation section.
According to the Food and Drug Administration (FDA), design outputs are the results of a design effort at each design phase and at the end of the total design effort. Similar to design inputs, highly regulated industries oblige manufacturers to establish and maintain procedures around design outputs. These procedures, along with items such as specifications and drawings are examples of design outputs. More on design outputs later when we discuss design validation.

Figure 3: Elements of the Design Validation Phase, required before proceeding to Development.
Equipment validation at this point may be a requirement for regulatory compliance. However, done correctly, it can be beneficial in preventing future costs for the manufacturer. Given that validation is essentially an evidence-based affirmation that the equipment can consistently and reliably achieve predetermined specifications, then a robust and documented validation should eliminate the need for in-process testing and controls.
The first step is to establish and document the intended use (user requirements). A risk assessment should then be completed prior to installation of the equipment per the required specifications. For off-the-shelf equipment, this is usually the manufacturer’s recommendations. At this point, the Installation Qualification (IQ) may be executed. This involves confirmation through objective evidence that the equipment has been installed and configured per specifications.
Like equipment validation, supplier evaluation and testing of incoming materials may be a legal requirement and again, if done correctly, can be a forward-looking cost prevention measure. At this point in the process, you've probably already determined the quality requirements of the product and identified material suppliers. If you are in a highly regulated industry, you need to certify these suppliers and implement a quality service level agreement to ensure incoming material meets a pre-defined standard. If not, it’s still good practise to evaluate your suppliers because it may reduce incoming defects and cycle times in the future.
Similarly, you may need to implement a receiving inspection process to test incoming material and verify the health of your supplier’s testing practises. Any material that fails to meet specification should be rejected and a corrective action plan agreed with the supplier.
We've already discussed testing and analysis in the design section. Now, we need to talk about test method validation because we need to confirm that the test method is suitable for its intended use. A typical test method will use equipment, materials and a testing process. For validation purposes, these all need to be included in a series of experiments using the given test method for routine sample analysis. The results of these experiments are statistically analysed to determine if they can consistently and reliably achieve predetermined acceptance criteria. If they can, then the test method is deemed to be valid.
This section concerns itself with testing the critical design outputs and ensuring that they meet the acceptance criteria. It can sometimes be difficult to determine which design outputs are deemed to be critical. One way is to ask yourself if a given output is essential for the product to function correctly? If so, then it's likely to be a critical output.
In summary, design output testing is ensuring that the product specifications meet user needs and intended use. Testing here is a collective term for testing, inspection and analysis. Although there can be similarities, it is different to the overall product/process validation which we will get to later. At this point, only to design is being tested and not the process.

Figure 4: Elements of the Development Phase, required before proceeding to Product/Process Validation.
Pilot manufacturing may begin prior to, or overlap with, the design validation stage. Pilot manufacturing is when the product design is transferred into manufacturing specifications, basically making it the start point for full scale manufacturing. In some companies, this phase will be the handover point from R&D to the commercial manufacturing team.
The benefit of manufacturing on a pilot scale is that it minimises risk. Pilot manufacturing often exposes potential difficulties in the process that would be very costly if they were not found until later. Examples include quality, efficiency, material yield and cycle time issues. Do you really want to be finding out about a major issue in one of these areas at the commercialisation stage? Informing top level management (or maybe even the customer) of that would be quite the difficult conversation!
The structure, efficiency and capability of any manufacturing process will impact upon the cost of that process and the quality of product that results from it. Therefore, good process is key to reduced cost and better product quality. Processes can be improved upon at any point in their lifecycle through the practical application of modern-day quality management programs such as Six-Sigma. So, if you have encountered issues, potential issues or simply found opportunities to optimise the process during the pilot manufacturing stage, then now is the time to make those changes.
Production ramp is when a company increases its production, typically ahead of anticipated demand increase. Sometimes, as is the case here, this will be prior to validation and commercialisation of a new product. This is not something that should be underestimated and planning should commence in plenty of time (don’t wait until after the pilot manufacturing step). Ramping up production requires consideration of many items and ensuring that there are enough materials, equipment, tooling etc available two facilitate the ramp. Most importantly of all, you need to ensure that you have the right people with the necessary skillset in place
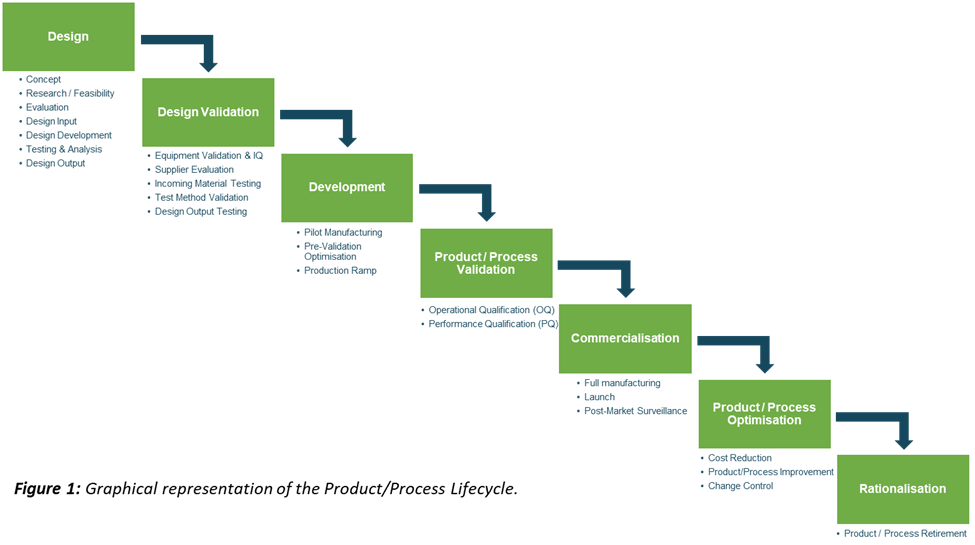
Figure 5: Elements of the Product/ Process Phase, required before proceeding to Commercialisation.
The Operational Qualification (OQ) phase is where you verify and document that the manufacturing process is achieving its operational requirements (i.e. within the predefined operating ranges). This is achieved via random testing from a series of manufacturing builds, where the upper and lower extremities of the in-process specifications were used. The outputs of the testing are statistically analysed to determine if they can consistently and reliably achieve predetermined acceptance criteria. If they can, then the OQ is deemed to be successful.
The Performance Qualification (PQ) is the final validation phase, where you verify and document that the manufacturing process is performing as anticipated when operating in conditions similar to what is expected during regular, commercial manufacture. Again, this is achieved via random testing from a series of manufacturing builds except this time, under normal operating conditions. Similar to OQ, the outputs of the testing are statistically analysed to determine if they can consistently and reliably achieve predetermined acceptance criteria. If they can, then the PQ is deemed to be successful.

Figure 6: Elements of the Commercialisation Phase, required before proceeding to Product/ Process Optimisation.
Earlier, we discussed ramping up production as part of the development phase prior to validation. Reaching full manufacturing is simply the result of that. You are now either operating at the capacity you wish to operate at or you’re operating at maximum capacity if you've run out of resources, floor space etc.
Product launch means different things to different people. For most, the phrase probably conjures up images of Apple’s iPhones or other hi-tech gadgets. Perhaps you think about marketing and attracting customers? Yes, that’s all part of it too. However, for the purposes of this paper, we will focus on the activity that needs to happen internally within the commercial manufacturing team prior to a launch.
A product launch is a process, not to be considered as an isolated event. It needs to be a planned and coordinated effort. Everybody within the company needs to know about the new product and understand the criticality of it. A product launch plan detailing the sequence of events, timelines and milestones is a must. Planning should start well in advance and allow plenty of time to rectify anything that might go wrong, without impacting the launch date.
Regarding manufacturing/processing of the product itself, additional diligence is required. Remember, this is a new product going to market and first impressions will matter. It is important that everybody involved in the building of the product along with those involved with its release are giving it due care and attention. After all the hard work to bring it this far, getting it wrong at this point could be detrimental to the future of the product.
Post-market surveillance is the structured monitoring of safety and performance of the product, following its release onto the market. Monitoring is performed by the manufacturer and achieved through proactively collecting and reviewing data. For safety reasons, it is a legal requirement in the pharmaceutical and medical device industries. This is because monitoring the product in the real world, using actual evidence from a larger population, offers far more data/information than what was possible to collect previously, therefore providing a more complete picture.
While it may not be a legal requirement, this practise is also recommended across other industries as a means of getting feedback on product performance. In fact, collecting and reviewing data throughout a product’s lifetime in the market should:

Figure 7: Elements of the Product / Process Optimisation Phase, required before proceeding to Rationalisation.
Identifying, quantifying, prioritising and planning cost reduction opportunities for your product is important to remain competitive. Typically, the value of a product will reduce over its time on the market. Therefore, to maintain margin, you need to reduce the cost of getting that product to the market.
There are many ways to approach this and we recommend that you read our white paper on it. However, we have given a taste of some of the opportunities below:
Previously, we looked at Pre-Validation Optimisation. At that point, we weren't discussing full commercial production. However, the basic principle remains the same, “The structure, efficiency and capability of any manufacturing process will impact upon the cost of that process and the quality of product that results from it. Therefore, good process is key to reduced cost and better product quality. Processes can be improved upon at any point in their lifecycle through the practical application of modern-day quality management programs such as Six-Sigma”.
The fact that we're now at a different point in the overall product/process lifecycle is not relevant. If you are encountering issues, potential issues or finding opportunities to optimise the process with a tangible return on investment, then make those improvements. Some general examples of how this can be done include:
Change control is critically important when optimising your process. At its worst, failure to manage change can result in a product or process operating outside of its validated range, leading to quality and compliance issues that could be ruinous for the business. While things may not get this bad, it is true that change done poorly can bring disruption to an organisation at high financial cost. This will often be direct (e.g. causing production down situations) but can also be indirectly affecting productivity and morale through the irritation and resentment of failure.
In summary, change is necessary for a business area to stay current and remain relevant but it needs to be carefully managed so that it is understood, planned, implemented and communicated in a structured and seamless manner.

Figure 8: Rationalisation – the final stage of the product / process lifecycle.
The final stage in the product life cycle is product rationalisation. Product rationalisation is reducing the number of products or stock-keeping units (SKUs) that a business manufactures or produces, either because they are negatively contributing to strategic goals or because the business wishes to invest in its more lucrative products. Simply put, it is the retirement of products or SKUs.
In many companies, products at the end of their lifecycle are not managed with the same degree of focus and attention as they are during other stages of the cycle. This is a failing and should be addressed because the protracted life span drives up cost via:
In order to remain competitive in the ever-evolving and challenging business environment, companies need to deliver year-on-year cost savings that are sustainable and don't negatively impact on company performance. However, this is easier said than done. There are a lot of considerations when approaching a cost reduction journey, namely what do you focus on?
A good starting point in answering this question is to understand what your business is trying to achieve in the medium to Long-Term. Does your organisation have a vision statement accompanied by a good strategy to realise this vision over the next 3 to 5 years? If so, this should dictate the level of cost reduction activity. For example, if your product is luxury/bespoke and high margin (e.g. Rolls-Royce), then strategically, cost reduction will not feature as substantially as quality or customer service. Conversely, for a low margin commodity product where cost competitiveness is critical (e.g. baked beans), you will typically see unit cost as a leading item in the business strategy.
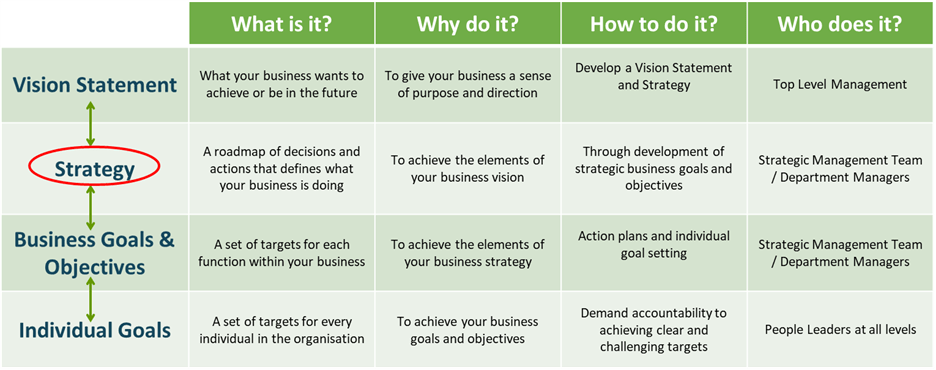
Figure 1: Unit Cost is typically part of the business strategy of a company where cost competitiveness is critical.
Therefore, prior to attempting a cost reduction journey, it is important that your company has a vision statement and that unit cost is appropriately allocated to the business strategy. Without it, misalignment may occur in relation to where cost reduction ranks relative to other priorities. This can result in focus in the wrong areas and failure in all!
Now that we have addressed the relationship between business strategy and unit cost, the remainder of this paper will assume that aggressive cost reduction is indeed part of the strategy of our hypothetical company. From Figure 1 on the previous page, we know that the next step is to set business goals and objectives. However, the difficulty is that that you may not have existing cost saving ideas for the purpose of setting these goals. Figure 2 lists a number of high-level suggestions to get started. Although categorised for simplicity purposes, it is important to know that the categories are not mutually exclusive. Overlap is recommended for your business. These suggestions are explained further in the following pages.
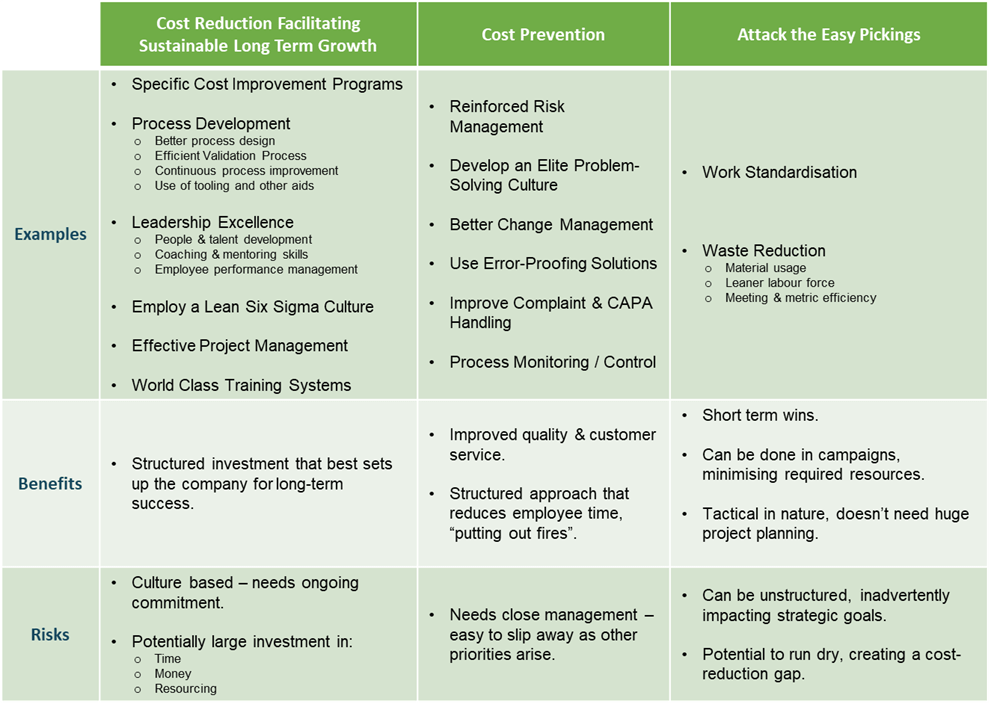
Figure 2: Categorised cost improvement examples with potential benefits and risks.
Referring back to Figure 1, once our strategy has been agreed and we’ve selected the cost reduction opportunities that we wish to pursue, the next step is to capture these in our business goals and objectives. Business goals are what the business intends to do and when they plan to have it done by. Business objectives are specific results that the business wishes to achieve. Most top modern-day businesses tend to encapsulate both goals and objectives together using the SMART format. SMART is an acronym for Specific, Measurable, Achievable, Relevant and Timebound. The benefit of using the SMART format is that goals tend to be clearly defined and well thought out, providing a sense of clarity and direction that makes success more likely. Figure 3 below defines SMART goals.
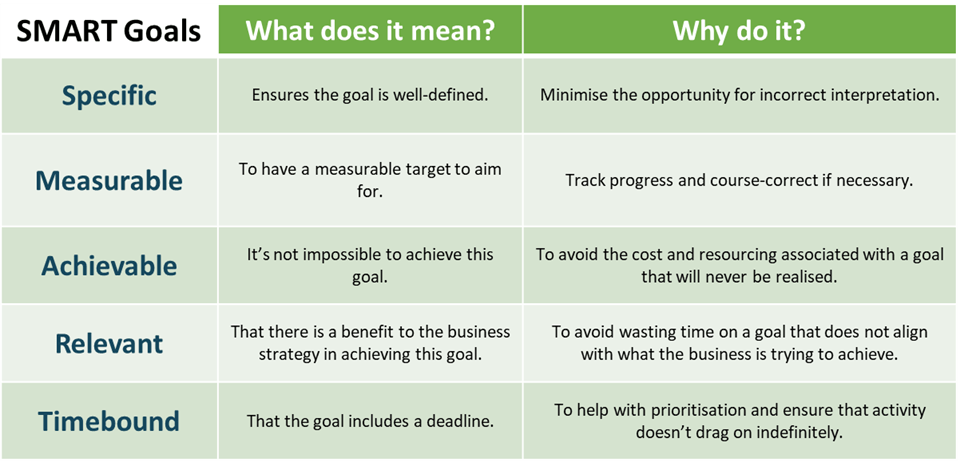
Figure 3: SMART Goal definition.
Below are good (SMART) and bad examples of a goal for a manufacturing company that has called out a reduction in variable overhead costs as part of its strategy:
As you can see, there is a big difference in the clarity and direction of the SMART goal. The SMART goal is specific, calling for a yield increase and specifically calling out aluminium material and the beverage can line. It is measurable – needs to move to 95%. It is achievable (not looking for zero scrap or a yield greater than 100%). It is relevant because material is a variable overhead cost, something the company wishes to reduce as part of its strategy. Finally, it is timebound - needs to be done by 30th June 2022.
Figure 4 below lists some of the mistakes that a company can make while attempting to cut costs. While some of these measures may seem appropriate at face value and may even work in short bursts, by their nature they are unstructured and the results can be unintentionally detrimental to the business.
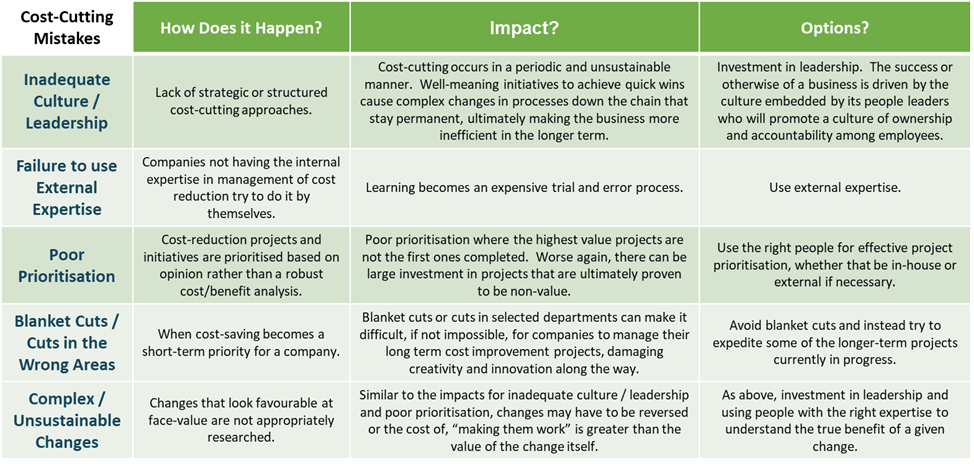
Figure 4: Cost-Cutting Mistakes.